In this article, I shall delve deeper into the main concepts for the particulate model of matter – taught at lower secondary science level.
When I started this blog, I wanted to share my experiences teaching, learning in this journey and at the same time, pen down some of the more recent thoughts that were running through my head. Therefore, I shall be sharing more about how I analyse information in the context of academics (mainly sciences) and break down information into bite-sized content that is easy to understand.
On its own, science can sometimes seem rather boring when it feels very content- heavy, and so I was looking to find a way to explain concepts in a way that was fun and appealing. Let me know what you think! 🙂
The Particulate Model of Matter (Brownian Motion & 3 States of Matter)
In this topic, the few main concepts we will look into are:
- Brownian Motion/Kinetic Particle Theory↯
- 3 States of Matter
- Effect of temperature on the motion of matter🌡️
- Applying ChatGPT as a learning resource🤖
Before we move into this topic, perhaps it is good to also revisit concepts that you have picked up in the past.
- All Matter have mass & occupies space
- The Three Types of Matter are Solid, Liquid and Gas.
- Characteristics of Solid vs Liquid vs Gas

How I will go about this is to picture a few different scenarios and put together some analogies, which I hope can help to bring some of the concepts to life. Do take note that this is only a reference, and it might not be as relatable to you – but I hope it serves as inspiration for you to find your own cadence and style of learning that suits you!
Here is an article about utilising different learning style to enhance your learning:
Brownian Motion/Kinetic Particle Theory↯
Brownian Motion/Kinetic Particle Theory highlights the theory that particles are in constant & random motion due to the uneven distribution of particles (in “zig-zag↯” patterns)
This relates to the idea of the particles constantly moving around, colliding with one another.


You can picture the bumper cars as the individual particles within matter, being in constant and random motion, after colliding with one another, or with the walls of the bumper car arena.
What is important to note is that matter consists of tiny particles that are invisible to our eyes (but could be seen with the help of a microscope) and what allows it to be in constant and random motion is due to the kinetic energy it possesses.
The Particulate Model of Matter
While reviewing the 3 states of matter below, some things to consider when differentiating them are:
- Attractive Forces between the particles
- How are they packed?
- Arrangement of Particles
- How the above mentioned factors affect the motion of the particles
Solids🍫, Liquids💦, Gases💨
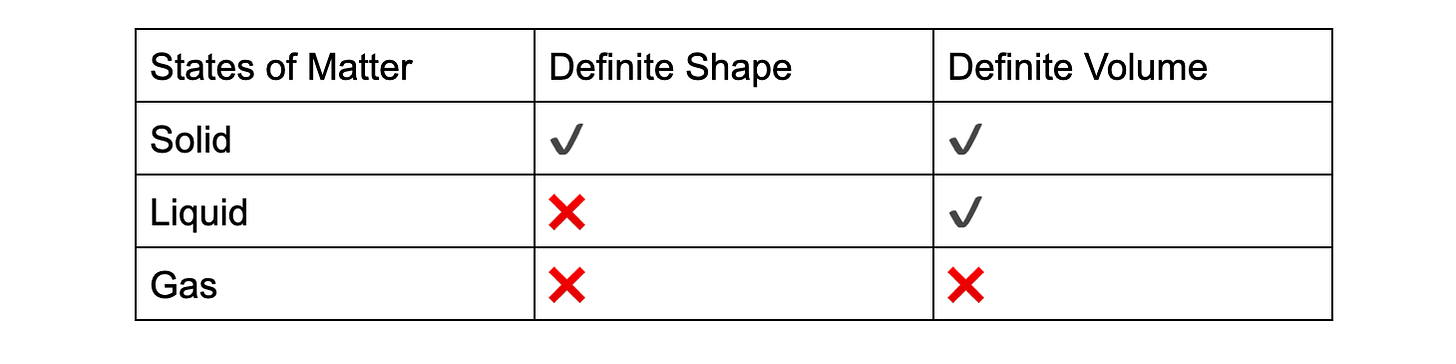
For Solids, how you may picture this is to consider what solids look like. If you recall what you have learnt previously, solids have a definite shape and volume. Looking at these diagrams here, you can relate solids to many different things that you may find intriguing and relatable: a bar of chocolate, soldiers standing in order,etc.

Main Characteristics:
- Very Strong Attractive Forces between particles
- Arranged in an orderly fashion
- Tightly/Closely Packed
- Vibrate in fixed positions


For the arrangement of solid particles, you can consider them just like soldiers in a file – orderly/regular.


As you can see, this chocolate bar is quite tightly packed and has a uniform appearance.
This can be used as reference for solids, which are very closely packed together due to strong attractive forces holding them together, which allows them to only vibrate at their fixed positions. This also contributes to the high density that solids have – due to the high proportion of particles to space (High mass per volume ratio).
Moving back to what we learnt before, solids have a definite/fixed shape and volume. It has a fixed shape and volume because the particles are closely packed and vibrate in fixed positions. It cannot be compressed easily because of the same reason – there aren’t sufficient gaps within the particles to compress them any further.
Be Like Water 💧, My Friend
– Bruce Lee


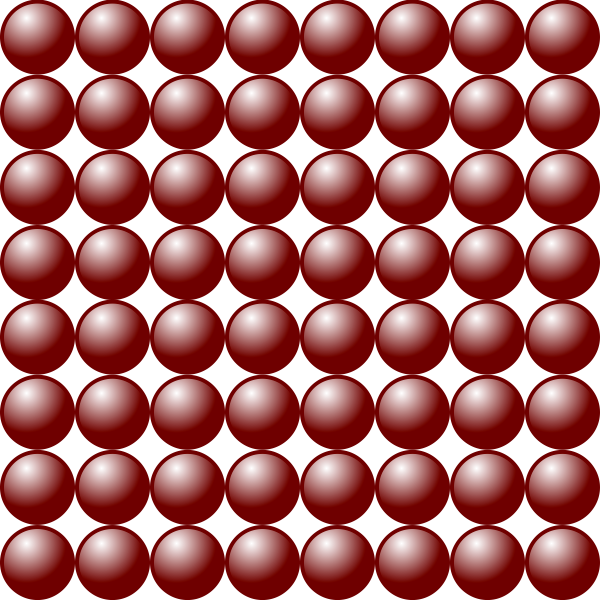
For Liquids, they are less uniform and have a slightly larger range of motion than Solids.
Main Characteristics:
-
- The attractive forces between liquids are moderately strong (weaker than solids which are more tightly packed)
-
- The arrangement is rather disorderly/random
-
- The particles are close together
-
- Particles can move about freely by sliding over one another in confined space


Source: Lord Koxinga, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons
How you can remember the model of matter for liquids is to picture the traffic of a bustling city.
Many vehicles line up at the red light – cars, trucks, motorcycles, etc.
The arrangement is disorderly & random because of the different sizes of the vehicles and the way the vehicles line up. During a traffic congestion/peak hour traffic, thereis little space for the cars and they are relatively close together (but not sticking to each other).
This explains how liquids have moderately strong forces of attraction between the particles, but not as much as solids where particles are packed very tightly together – therefore it has a definite volume as the forces are strong enough to hold the particles together in a fixed amount of space.
However, the flow of traffic changes as the vehicles move along, with some motorcycles/cars weaving in and out of traffic. – This is similar to how liquid particles slide over one another in confined spaces.


Source: Dale J. Brugh, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons
Liquid does not have a definite shape due to the motion of liquid particles – where they can move about freely by sliding over one another in confined spaces, and will take the shape of the container.
Main Characteristics:
-
- The attractive forces between gases are weak or close to zero (negligible)
-
- The arrangement is disorderly/random
-
- The particles are far apart from each other
-
- Move freely in ALL directions at high speeds
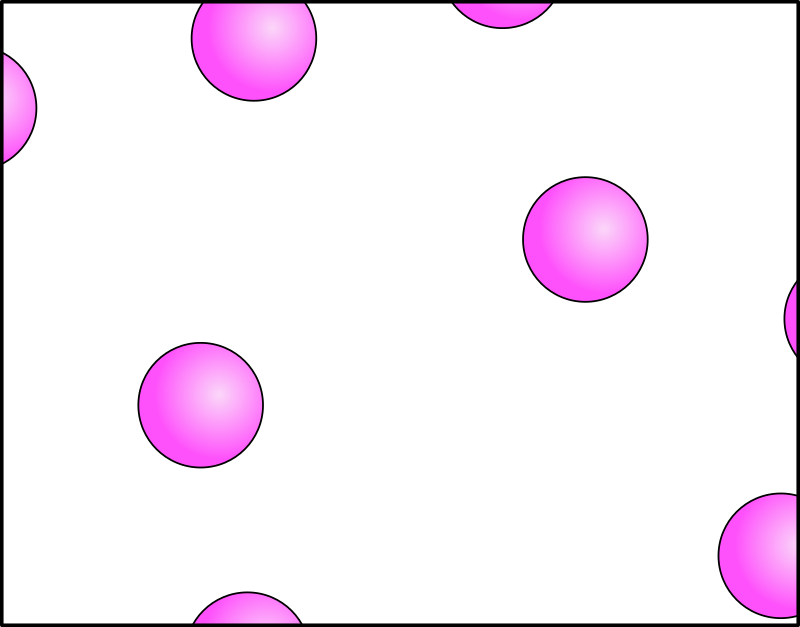



Using balloons in a room as an example:
Random motion – When you release a number of balloons in a room, it moves randomly, bouncing off one another & colliding with the walls of the room
Diffusion – Gases can diffuse easily, just like how balloons are free to move anywhere in the room at high speeds.
Can be compressed easily – After releasing the balloons in the room, you can collect it back after and squeeze it into a bag. Or you can consider how it is possible to push the balloon closer together in one corner of the room to be more tightly packed.
For gases, as you may recall, gases do not have a fixed shape/definite volume, the reason being that it is spread very far apart – so they are free to move randomly in ALL directions. Gases can be compressed because the distances between the particle is huge – so when a force/pressure is applied (for example: depressing a syringe), the particles can be pushed closer together, occupying less space/volume.
Effect of temperature on the motion of matter🌡️
The first thing we must understand is that temperature is used to measure the average kinetic energy particles have. When you increase the temperature of something, you are introducing heat energy to a substance, which is converted to kinetic energy of the particles within the substance.
So, what happens when you increase the temperature/heat up a substance?
Heating♨️
As the temperature of a substance increases, the particles gain heat energy and thus kinetic energy, vibrating more vigorously, increasing the space/distance between particles, resulting in expansion of the substance.
This is a phenomenon you may observe in balloons – when you introduce heat to a balloon, the rubber will stretch and the air inside will expand as it gains heat.


This is also why train tracks have multiple gaps between them – to accommodate for expansion in volume and to prevent buckling of the train tracks.
To simplify this into an analogy, you can picture a hot pot with ants crawling on top 🥵, scrambling to run away from the heat. (In Chinese, this is referred to as: 热锅上的蚂蚁).As the pot gets warmer and warmer (heat energy/temperature), and the ants will start moving more frantically. (kinetic energy)
Cooling🧊
This is just the opposite of heating. If heating causes expansion, cooling causes contraction.
This is because particles lose kinetic energy during cooling, therefore their motion decreases, causing them to be packed more closely together. → You can also make a connection of this concept to membrane fluidity of a cell (biology)
How you can visualise this is by imagining a colony of penguins during the cold months of winter: As the winter draws on, the penguins will huddle together to keep warm (particles come closer to one another 😛)


Applying ChatGPT as a learning resource🤖
If you are looking for a way to combine all three states of matter together, check out how you can relate it to a party (generated by ChatGPT)!


Sometimes it can be rather long-winded, but I think it could help for concepts that are difficult to understand and digest. If you are interested to find out how you can utilise Analogies to learn more effectively, check out my other blog post below:
Concluding Thoughts
All in all, I hope this quick guide helped you to understand more about the particulate model of matter, and how the movement of the particles are involved in the process. 🙂 Let me know if you require any clarifications, or if there are areas you would like me to improve on.
Happy reading ~
