In this article, I shall cover the basics of atoms, ions and molecules in lower secondary science: chemistry🧪.
Read on to find out more!🚀
Table of Contents:
- Atoms
- Introduction to Periodic Table of Elements
- Electronic Configuration
- Isotopes
- Ions
- Molecules
- Concluding Thoughts
Atoms
Atoms are the smallest particle of an element. An atom contain a nucleus – which contains protons and neutrons, as well as electrons which are found on the electron shells (outer layer of the atom).

It kind of looks like Saturn, with the outer rings being similar to the electron shells, and the planet core looking like the nucleus.
The Three Constituents:
Protons🙂 – These are the particles within the atoms which carry a positive charge.(as you can infer from the name, it starts with P)
Neutrons😐 – Neutrons do not carry any charge (Neutrons = Neutral)
Electrons😢 – Electrons carry a negative charge. Electrons are extremely important in determining chemical properties, as they decide how the atoms of same/different types are chemically joined together.

Source: AG Caesar, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons
You can think of the nucleus being the house of the atom – trying to maintain and keep the positivity within the house, while keeping the negative electrons on the outside.
Do take note that for atoms, they are all about balance, so the number of protons (positive) balances out with the number of electrons (negative) such that atoms are electrically neutral/uncharged. – kind of like Yin and Yang

This is a table that you might have come across already when studying this topic, which details the relative mass and charge of the three different constituents of the atom.

Introduction to the Periodic Table of Elements
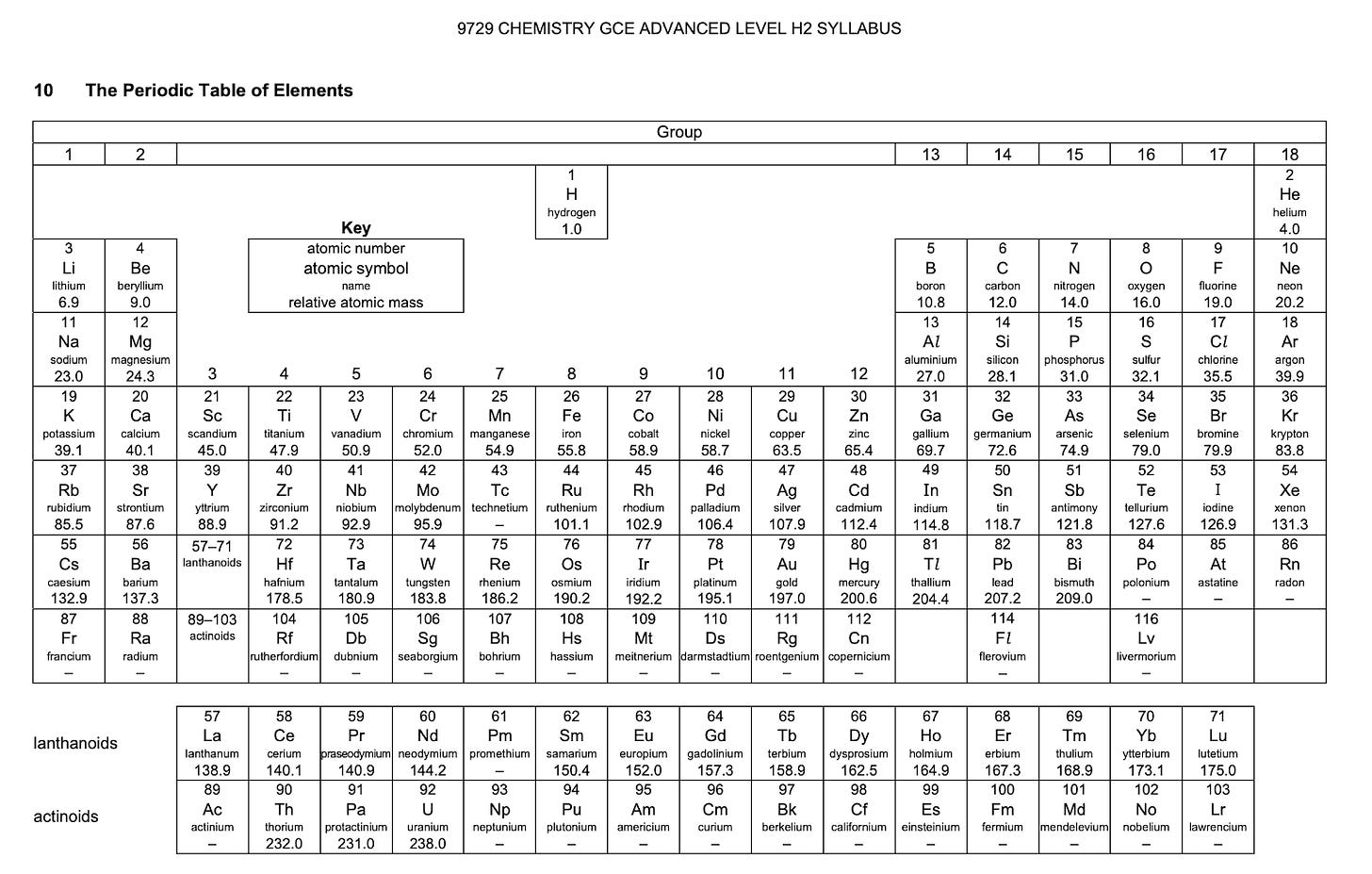
Group Number –
This refers to the column that the elements are in, from left to right. Different groups have different properties, for example, Group 1 on the utmost left are your alkali earth metals and they are very volatile and flammable – combust easily when reacting with oxygen/air
Or group 18 – Noble Gases → stable and inert (unreactive), as they already have a fully filled electron shell/stable electronic configuration, hence they are less affected by other elements.
Period Number –
This refers to the row that the element is in. In the top row (period 1), we have hydrogen and helium. In the 2nd row (period 2), we have lithium, beryllium, boron, carbon, etc. In the third row (period 3), we have potassium, calcium, etc.
Atomic Number –
This takes reference to the number of protons within an atom. So how you can understand this is that the atomic numbers are like the numbers that differentiates all the different types of atoms/elements within the periodic table. (just like the players in squid game🦑) The atoms are arranged based on their atomic number, from left to right (across the period).
Each element is labelled with a specific number –
Hydrogen with 1 Proton, Helium with 2 Protons, Lithium with 3 Protons etc.
Atomic Mass –
Based on the periodic table above, this is labelled as relative atomic mass.
Why is it called relative atomic mass? That is because it is the average mass of an atom – taking into account the different isotopes of the element. The relative atomic mass is based on the ratio of an element’s mass to 1/12 of a C12 Atom (which is basically 1).
Mass Number refers to the total number of protons and neutrons in the nucleus/atom combined. Mass Number is usually the one that is heavier (except for hydrogen), and will never be smaller than the atomic number (number of protons only).
Electronic Configuration
As the name suggests, electronic configuration refers to the configuration/arrangement of electrons in an atom. The electrons are actually arranged in electron/orbital shells, with the innermost electron shell also housing 2 electrons, followed by 8 electrons in the 2nd electron shell, 8 electrons in the 3rd electron shell, 18 electrons in the 4th electron shell, and so on…
(Electronic structure: 2.8.8.18)
At this stage, what we need to familiarise with is how do we go about drawing the electronic configuration of the different atoms?
For hydrogen and helium atoms, they are both in period 1. They only have one electron shell that houses up to two electrons, as a hydrogen atom only has 1 electron and a helium atom has 2 electrons.
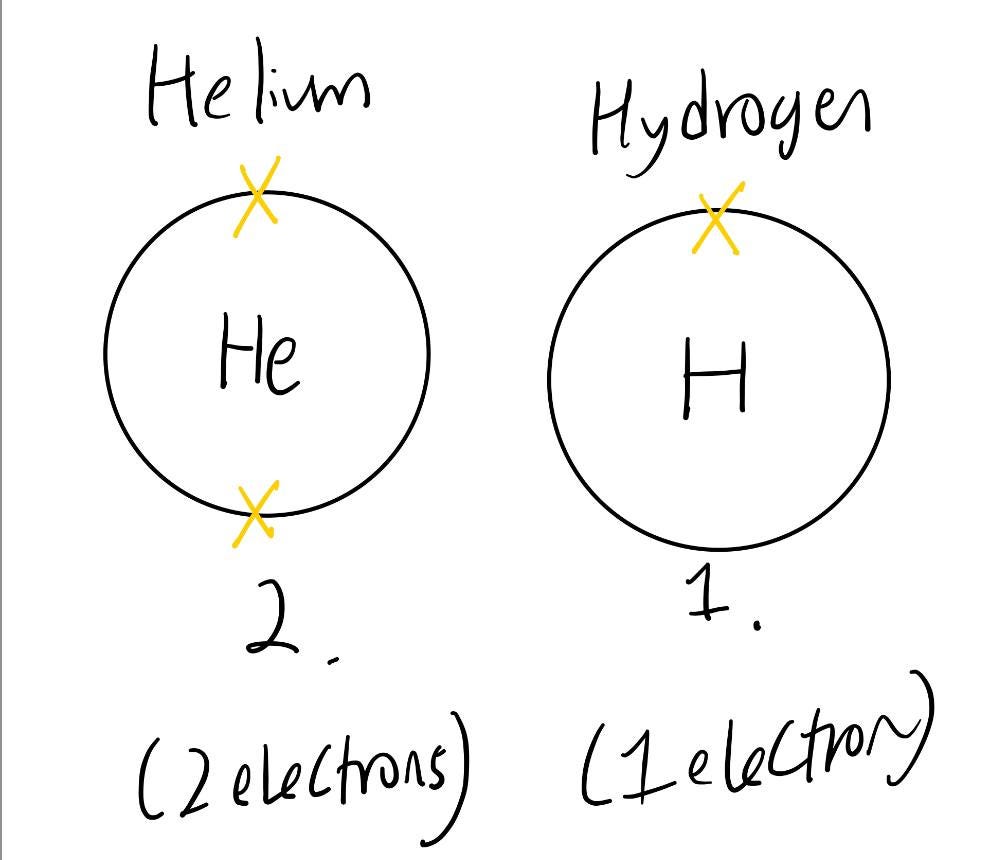
As we move on to elements in period 2, they have more than two electrons. We have to fill in the innermost electron shell first (housing 2 electrons), before we move to the next electron shell on the outer layer (housing 8 electrons).
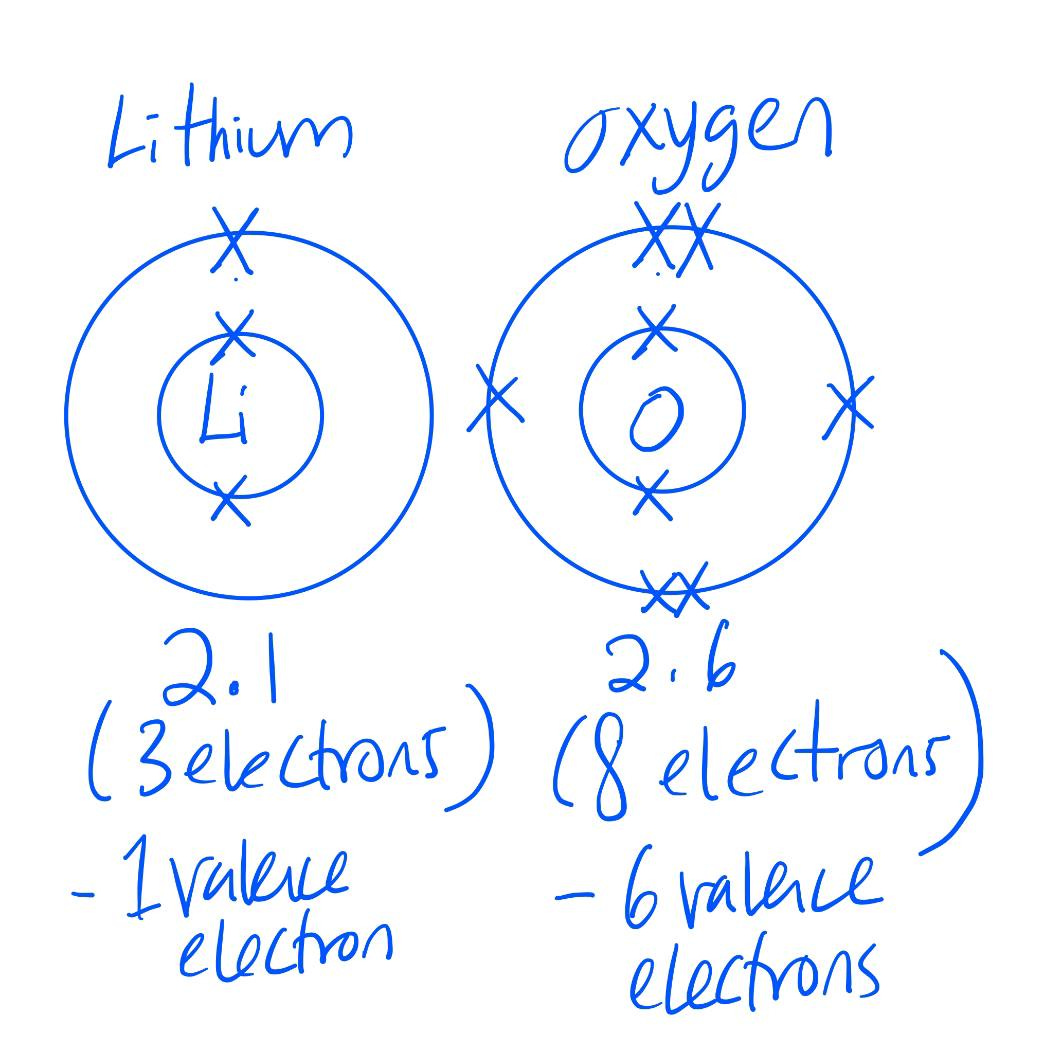
Same goes for elements in period 3:
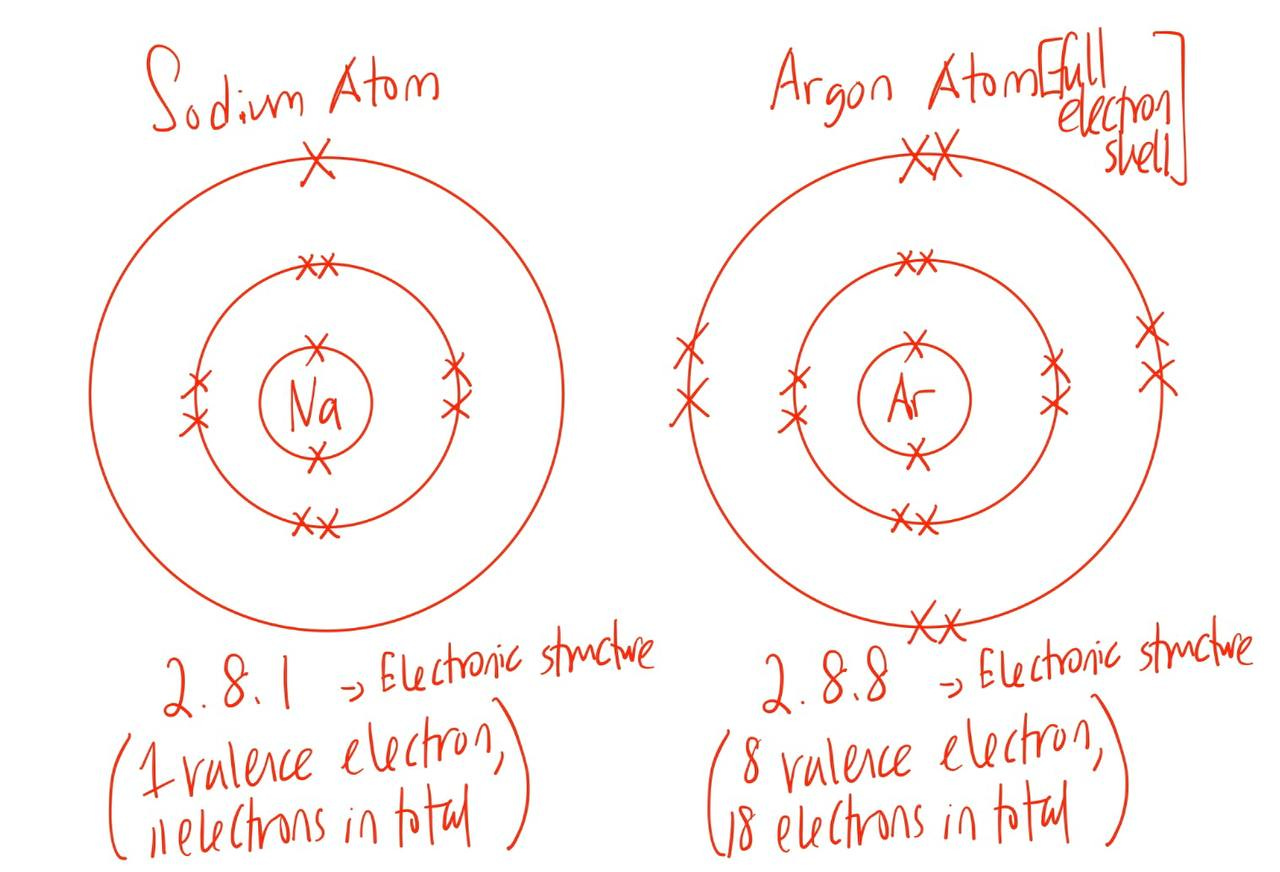
Isotopes
Isotopes are a subset of Atoms. This is when atoms have different numbers of neutrons but the same number of protons and electrons.
How you may remember this is by first looking at the word isotopes – iso stands for the same, equal.
As atoms are uncharged, isotopes also have an equal number of protons to electrons.
Source: BruceBlaus, CC BY 3.0 <https://creativecommons.org/licenses/by/3.0>, via Wikimedia Commons
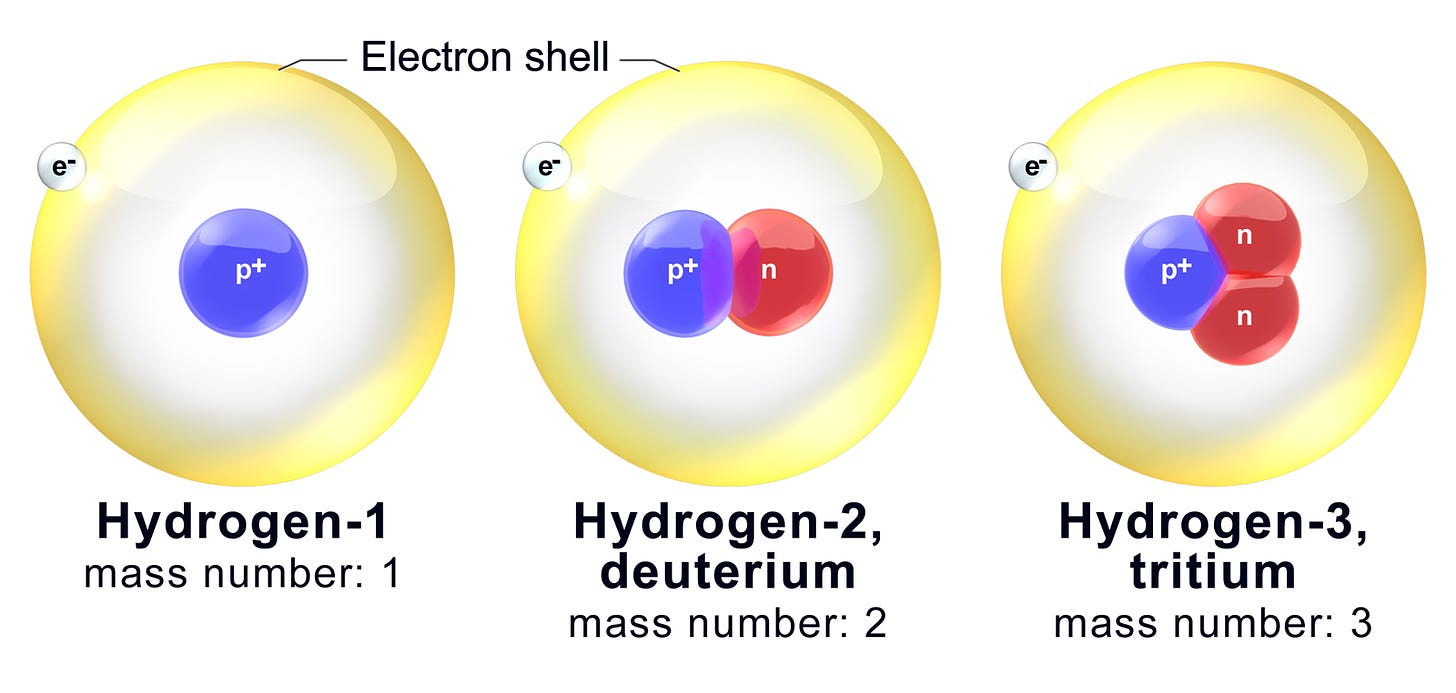
How I remember isotopes is by referencing one common example of an isotope, which is the isotopes of hydrogen.
The Hydrogen atom we normally see on the periodic table is hydrogen-1 (protium) in the image above, where there is only one proton in the nucleus, therefore it has a mass number of 1.
Hydrogen-2 is an isotope known as deuterium. It has a mass number of 2 (one neutron on top of the 1 proton in the nucleus), and it forms “heavy water” with oxygen – D2Oinstead of H2O.
Ions
There are two types of ions: Cations and Anions.
Ions are basically atoms but with charges – either through adding/gaining or removing/losing electrons. How I remember what ions represent which can be by associating the names of cations and anions to positive and negative respectively.
Anions – the word is similar to “onions” which make you cry when chopping them, so you can think of anions as negative
Cations – there is “cat” in the name, so you can associate that with cations being positive, getting rid of all the negative energy/bad luck (electrons).

With reference to the periodic table, there are a few things to take note of within the periodic table.
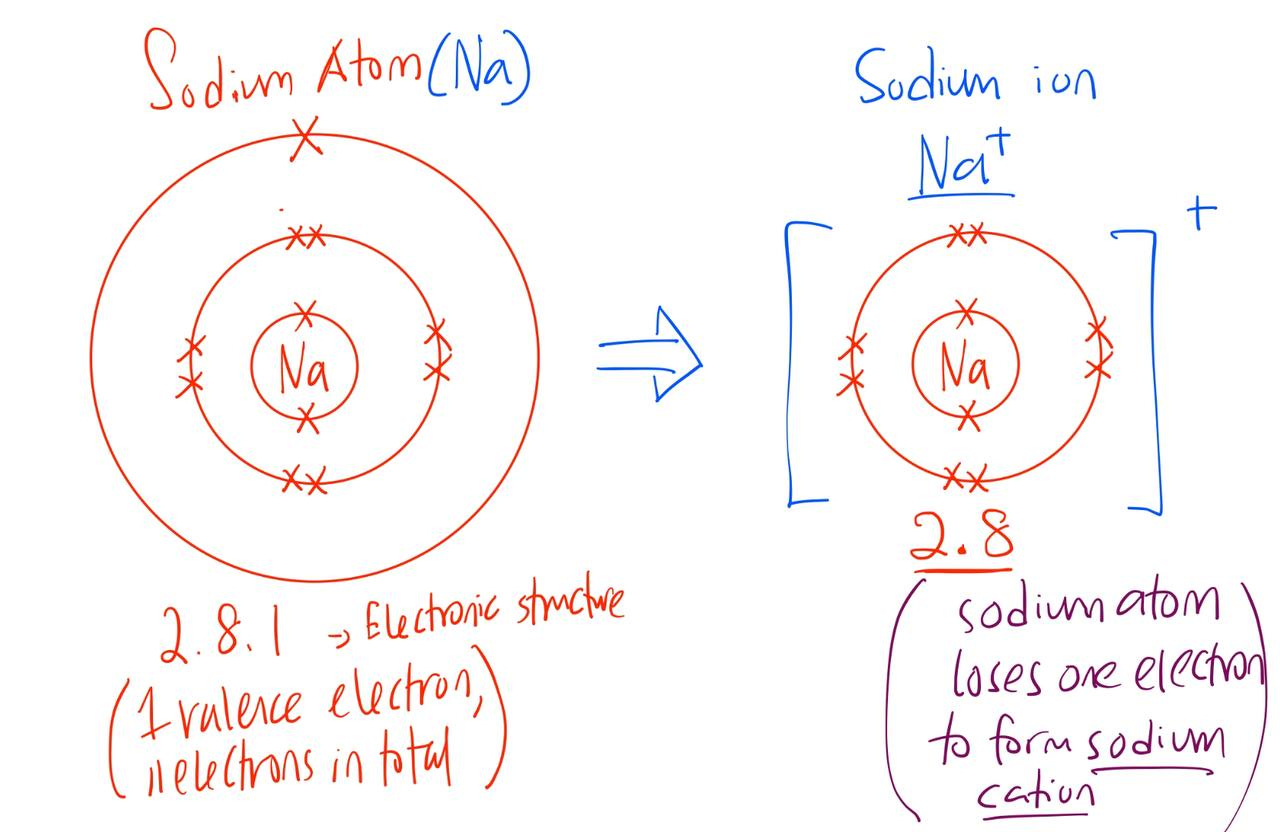
On the left hand side, you have your alkali metals. These are your sodium, potassium,etc.
These sodium, potassium atoms usually give out one electron to form a metal cation (positive ion).
For example, Sodium metal (Na) loses one electron to form Na+
This is also relevant when drawing out the electronic structure of the ion.
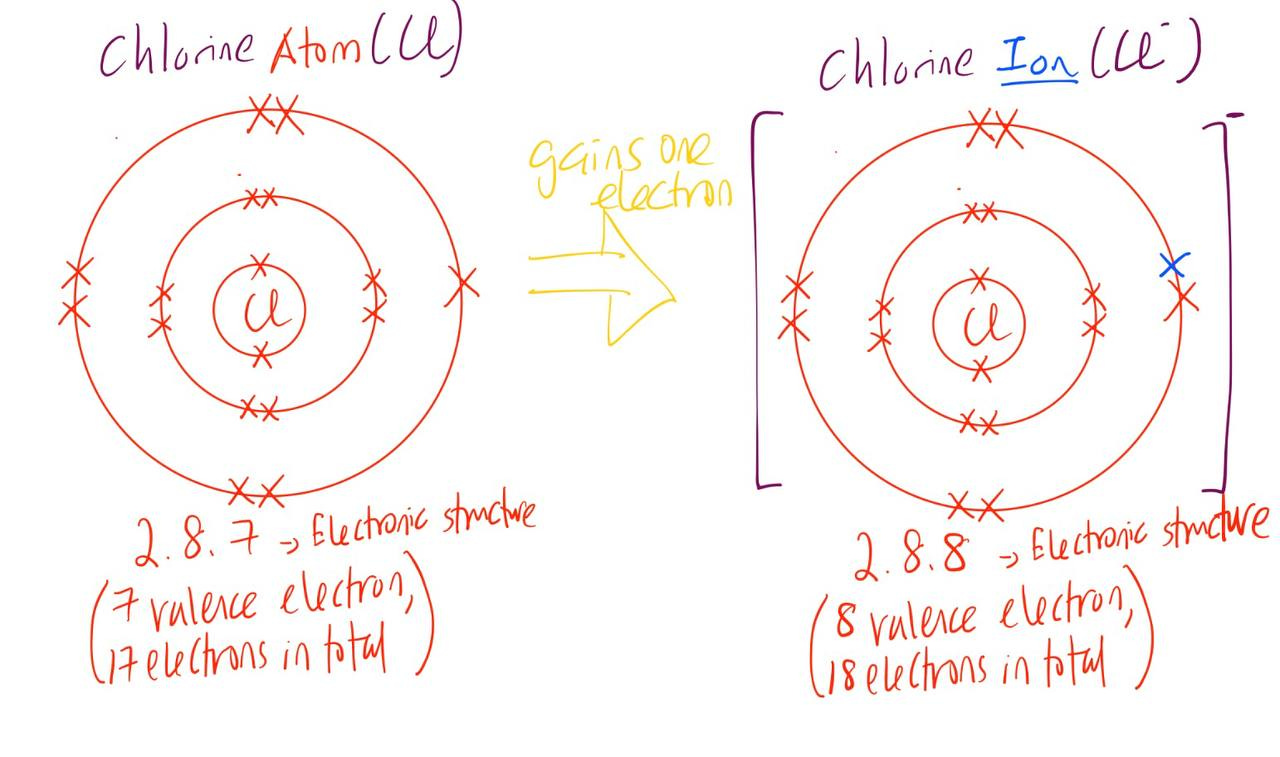
For example, when a chlorine atom gains an electron, it forms a chloride ion will a full octet structure/electron shell as shown above.
I will be drawing connections to some of the other topics that you might also learn along the way, so do not worry if you have yet to learn all the information given here yet 😅
For starters, a good rule of thumb to abide by is that metals usually form cations, while non-metals usually form anions.
Molecules
A molecule involves two or more atoms chemically combined together.
There are two different types of molecules:
- Molecules of Elements
- Molecules of Compounds
Molecules of Elements
Molecules of Elements are basically atoms of the same element joined together. Some common examples are Oxygen gas, Nitrogen gas, etc.
Test Yourself! Using the periodic table, identify these few gases:
- O2
- N2
- F2
(note: these are called diatomic molecules – where di stands for two)
Source: Christinelmiller, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons
Molecules of Compounds
Molecules of Compounds, on the other hand, involves atoms of different types/elements chemically joined together.
One of the most common examples that we use for this is water! The chemical formula for water is H2O → which stands for 2 Hydrogen Atoms that are chemically joined to an oxygen atom.
One way I suggest you to consolidate all this information is to draw connections between the different topics here – in a form of a simple mind map:
Check out how I did so in the article below!
Concluding Thoughts
Again, one of the ways that I recommend learning concepts that are new (and perhaps if you feel it is a bit dry) is to utilise different forms of multimedia – watching educational youtube videos – Crash Course, or searching in google where you might find images that help to sum up some of the more challenging concepts you might come across when studying.
While some of the online resources might provide too much information that you might feel too overloaded, or too little information where you might feel it does not provide sufficient advice, it is important to experiment with different styles of learning, finding ways to properly and efficiently process information covered in your syllabus. Check out my guide on using Mnemonics as a memory device to categorise challenging concepts and key words together! I do hope it helps 🙂
